
AFFILIAZIONE
insideai
AUTORE PRINCIPALE
PhD Coro Florinda
VALUTA IL CHALLENGE
Registrazione obbligatoria. Una valutazione consentita
login avvenuto
[ratingwidget]
GRUPPO DI LAVORO
PhD Coro Florinda insideai
Ing. Ravizza Alice insideai
PhD Hausner Sebastian university hospital würzburg
Prof. Pullig Oliver university hospital würzburg
Prof. Bloemen Veerle ku leuven
Prof. van Osch Gerjo erasmus mc, university medical center rotterdam
PhD Bonatti Amedeo Franco università di pisa
Prof. Weinans Harrie university medical centre utrecht
Prof. Zenobi-Wong Marcy eth zurich
Prof. Ulrich Inga university of zagreb
de Vivans Raphael efmc Prof. Vozzi Giovanni università di pisa
Ing. Ravizza Alice insideai
PhD Hausner Sebastian university hospital würzburg
Prof. Pullig Oliver university hospital würzburg
Prof. Bloemen Veerle ku leuven
Prof. van Osch Gerjo erasmus mc, university medical center rotterdam
PhD Bonatti Amedeo Franco università di pisa
Prof. Weinans Harrie university medical centre utrecht
Prof. Zenobi-Wong Marcy eth zurich
Prof. Ulrich Inga university of zagreb
de Vivans Raphael efmc Prof. Vozzi Giovanni università di pisa
AREA TEMATICA
Applicazioni innovative di bioingegneria: idee dalle Università
ABSTRACT
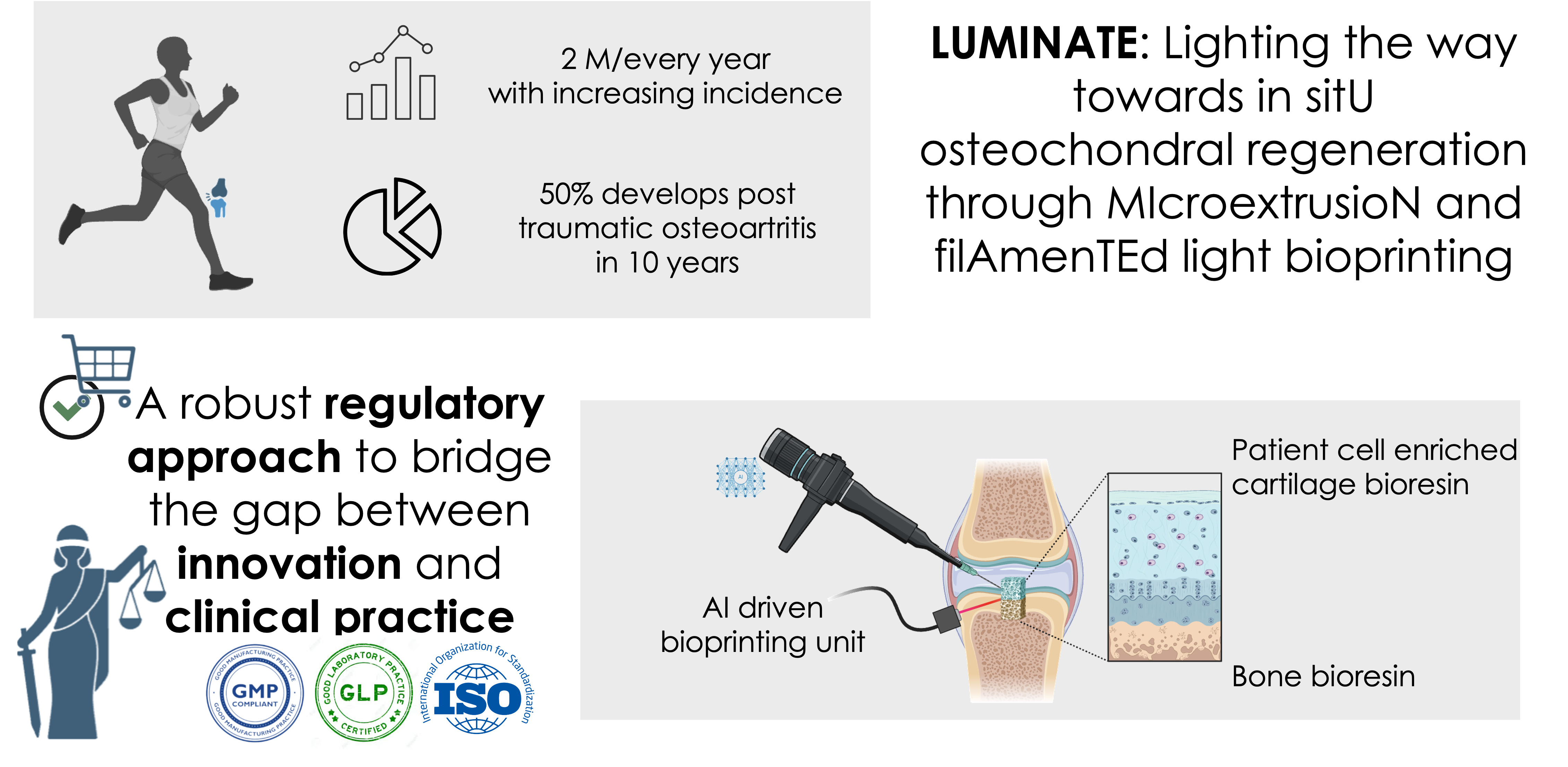
Traumatic cartilage injuries in diarthrodial joints, such as the knee, affect over 2 million people annually. Despite advances in treatment, many patients develop post-traumatic disabling osteoarthritis highlighting the urgent need for more effective solutions. The LUMINATE project proposes a one-stage, personalized treatment based on the deposition of photoresins filled with patient-derived cells through an AI driven bioprinting unit: Endoflight. The successful clinical translation of LUMINATE relies on a rigorous regulatory strategy to ensure compliance with the Medical Device Regulation (MDR), the Advanced Therapy Medicinal Products (ATMP) Regulation and AI Act: InsideAI srl participates to define such strategy. The LUMINATE bioprinting unit will require CE marking, demonstrating adherence to general safety and performance standards of MDR. The AI software for defect segmentation and material quantification is classified as medical device, requiring compliance with both MDR and AI act. From a qualification standpoint, the bioprinted constructs are regulated as ATMPs under European Medicinal Agency guidelines, due to the incorporation of viable cells aimed at regenerating damaged cartilage. When combined with a non-viable structure that provides structural support, the product is classified as a combination device. To ensure the safety and efficacy of the bioprinted constructs, biocompatibility testing will be necessary together with sterility assurance, and mechanical stability. For the cell-based components, strict GMP compliance is mandatory for cell isolation, expansion, and differentiation protocols, to ensure traceability and consistency. GLP standards will govern preclinical validation, the end point of LUMINATE project, including in vitro studies and large-animal models to evaluate the biological response, integration, and long-term functionality of the implants. To bring a medical device to market, it must undergo safety testing, preclinical trials on animals, and a series of extensive clinical studies, that are out of scope of the project. By including in the design pathway a robust regulatory approach, the LUMINATE project aims to set a new standard in personalized, minimally invasive cartilage repair, bridging the gap between cutting-edge innovation and clinical feasibility while addressing the growing medical and socioeconomic burden of post-traumatic osteoarthritis.
[flipbook pdf=”https://sem2000.s3.eu-west-1.amazonaws.com/OLC/0989478442373166690049893/scent/130350___M79RIyfo.pdf”]


